Hiperexpressão de Twist1 no carcinoma colorretal primário
Rúbia Denise Ruppenthal1; Ana Paula S. Fernandes1, 2; Jordan B. Santos1; Adriana V. Roehe2; Daniel C. Damin1
1. Universidade Federal do Rio Grande do Sul, Porto Alegre, Rio Grande do Sul, Brazil.
2. Universidade Federal de Ciências da Saúde de Porto Alegre, Porto Alegre, Rio Grande do Sul, Brazil.
J Bras Patol Med Lab. 2021; 57: 1-6.
DOI: 10.5935/1676-2444.20210060
ABSTRACT
Introduction: The Twist-related protein 1 (Twist1) is a transcription factor of the helix-loop-helix type whose high tissue expression has been observed in colorectal cancer (CRC) and other different neoplasms. It plays a central role in epithelial-mesenchymal transition and the mechanisms of invasion, metastasis, and acquired resistance to chemotherapy. Objective: To analyze Twist1 overexpression in CRC and its correlation with clinical pathological parameters. Methods: Twist1 overexpression was analyzed by immunohistochemistry in paired samples of tumor and non-tumor tissue adjacent to the tumor of 20 patients with CRC and the normal colorectal tissue of 10 patients without cancer (control group). Results: We observed that the Twist1 expression was predominantly cytoplasmic, with immunostaining variations regarding to extent and intensity. Twist1 overexpression was evidenced in 34 (68%) of the 50 samples analyzed. Twist1 overexpression is significantly higher in CRC and non-tumor tissue adjacent to the tumor compared to the non-cancerous colorectal tissue in the control group (p < 0.001), in which Twist1 overexpression was not detected. Twist1 overexpression is significantly related to higher-grade tumors in TNM staging (p = 0.038), but not to patient age or gender, nor tumor location and differentiation. Conclusion: Twist1 is expressed differently in patients with CRC and those in more advanced stages of the disease. These data suggest that Twist1 overexpression may be assessed as to its use as a possible tumor marker in the future.
Key words: immunohistochemistry; gene expression; colorectal neoplasms; Twist-related protein 1.
RESUMO
Introdução: A proteína 1 relacionada à Twist (Twist1) é um fator de transcrição do tipo hélice-volta-hélice cuja expressão tecidual elevada tem sido observada no carcinoma colorretal (CCR) e em outras diferentes neoplasias. Ela tem papel central na transição epitelial-mesenquimal e nos processos de invasão, metástase e aquisição de resistência aos quimioterápicos. Objetivo: Analisar a hiperexpressão de Twist1 no CCR e sua correlação com parâmetros clinicopatológicos. Métodos: A hiperexpressão de Twist1 foi analisada por imuno-histoquímica em amostras pareadas de tumor e de tecido não tumoral adjacente ao tumor de 20 pacientes com CCR e no tecido colorretal não maligno de 10 pacientes sem neoplasia (grupo-controle). Resultados: Observamos que a expressão de Twist1 foi predominantemente citoplasmática, com variações na imunocoloração quanto à extensão e à intensidade. A hiperexpressão de Twist1 foi evidenciada em 34 das 50 (68%) amostras analisadas; foi consideravelmente maior no CCR e no tecido não tumoral adjacente ao tumor em relação ao tecido colorretal não maligno do grupo-controle (p < 0,001), no qual não foi detectada. A hiperexpressão de Twist1 está significativamente relacionada com tumores em estágios TNM mais elevados (p = 0,038), porém, não com idade e sexo dos pacientes, nem com localização e diferenciação tumoral. Conclusão: Twist1 está diferentemente expresso nos pacientes com CCR e naqueles em estágios mais avançados da doença. Esses dados sugerem que a hiperexpressão de Twist1 deve ser avaliada quanto ao seu emprego como possível marcador tumoral no futuro.
Unitermos: imuno-histoquímica; expressão gênica; neoplasias colorretais; proteína 1 relacionada à Twist.
RESUMEN
Introducción: La proteína 1 relacionada con la torsión (Twist1) es un factor de transcripción hélice-bucle-hélice cuya alta expresión tisular se ha observado en cáncer colorrectal (CCR) y otras neoplasias diferentes. Desempeña un papel central en la transición epitelio-mesénquima y en los procesos de invasión, metástasis y adquisición de resistencia a la quimioterapia. Objetivo: Analizar la sobreexpresión de Twist1 en CCR y su correlación con parámetros clínico-patológicos. Métodos: La sobreexpresión de Twist1 se analizó mediante inmunohistoquímica en muestras pareadas de tejido tumoral y no tumoral adyacente al tumor de 20 pacientes con CCR y tejido colorrectal normal de 10 pacientes sin cáncer (grupo control). Resultados: Observamos que la expresión de Twist1 fue predominantemente citoplasmática, con variaciones en la inmunotinción en términos de extensión e intensidad. La sobreexpresión de Twist1 se evidenció en 34 (68%) de las 50 muestras analizadas. La sobreexpresión de Twist1 es significativamente mayor en el CCR y el tejido no tumoral adyacente al tumor en comparación con el tejido colorrectal no canceroso en el grupo de control (p < 0,001), donde Twist1 no detectó sobreexpresión. La sobreexpresión de Twist1 está significativamente relacionada con los tumores de alto grado en la estadificación TNM (p = 0,038), pero no con la edad o el sexo del paciente, ni con la ubicación y diferenciación del tumor. Conclusión: Twist1 se expresa de manera diferente en pacientes con CCR y en aquellos en estadios más avanzados de la enfermedad. Estos datos sugieren que la sobreexpresión de Twist1 puede evaluarse para su uso como posible marcador tumoral en el futuro.
Palabras clave: inmunohistoquímica; expresión génica; cáncer colorrectal; proteína 1 relacionada con la torsión (Twist1).
INTRODUCTION
Colorectalcancer (CRC) is the third most prevalent type of cancer in the world; it is responsiblefor a significant morbidityandmortalityrate(1) . Most patientsremainasymptomaticforseveralyears; they are commonlydiagnosed at advanced stage of the disease. However, whendiagnosed at early stage, CRC is a treatableandoftencurablecancer(2).
Twist-related protein 1 (Twist1) was recently reported as an important regulator of carcinogenesis and tumor metastasis in different solid tumors(3). It acts as a helix-loop-helix transcription factor; it is encoded by the Twist1 gene(4, 5). Ectopic expression of the Twist1 protein results in loss of cell-cell adhesion mediated by E-cadherin, activation of mesenchymal markers, and induction of cell motility(6). The expression of Twist1 induces the epithelial-mesenchymal transition (EMT) – necessary for the metastatic process –, thus contributing to tumor development and progression(7, 8).
In this context, studies have shown that the participation of Twist1 in different stages of the tumor process is directly related to its tissue overexpression(7, 9-12). Twist1 overexpression has a strong relationship with lymph node invasion, tumor progression and growth(13). In addition, there may be a decrease in the survival rate of patients with tumors that overexpress Twist1(14). The altered expression of Twist1 is not only limited to the tumor, it may also be present in the macroscopic and microscopic normal mucosa of individuals with different carcinomas, a fact that possibly indicates an early biological role of Twist1 in neoplastic transformation(9, 12). Regarding CRC, it was demonstrated that Twist1 is significantly overexpressed in tumor samples concerning the non-tumor tissue adjacent to the tumor, the expression was correlated with the presence of lymph nodes metastasis(15) and chemoresistance(16). The authors propose the Twist1 overexpression as a new tumor marker for malignancy and as a potential therapeutic target in CRC.
OBJECTIVE The present study was designed to evaluate the Twist1 overexpression in a series of patients with primary CRC when compared to a control group (without the neoplasm).
METHOD
Patients and tissues
Twenty patients with a confirmed diagnosis of primary colorectal cancer were included in the study. From these, 12 were women and eight were men (mean age 63.4 years, range 33 to 75 years). Individuals with familial adenomatous polyposis, hereditary nonpolyposis colorectal cancer, and inflammatory bowel disease were excluded. None of the patients received anticancer treatment before inclusion in the study. Complete information about past medical history, physical examination, and laboratory test results were reported by consulting the medical record.
During surgical resection, two tissue samples were collected from each patient with colorectal cancer: a tumor sample and a non-tumor colorectal tissue sample located approximately 10 cm from the tumor. After histopathological examination of the specimens, the pathological stage was determined according to the TNM System of the AJCC(17), and were distributed as follows: three patients in stage I; five in stage II; eight in stage III; and four in stage IV. Eighteen tumors were histologically diagnosed as moderately differentiated cancer and two as poorly differentiated cancer.
As a non-malignant control group, 10 patients undergoing surgical resection for the treatment of benign intestinal diseases were included. This group consisted of five women and five men (mean age 51.7 years, range from 25 to 73 years). Tissue samples were obtained from a central area of the resected specimens. Histological analysis of the specimens confirmed the absence of malignant or pre-malignant lesions in the collected samples. After collection, samples were immediately immersed in 10% formalin and sent for immunohistochemical analysis.
The present study was approved by the Research Ethics Committee of the hospital where the study was carried out (Conep registration number: 1672000100005). Informed consent was obtained from all patients before inclusion in the study.
Immunohistochemistry
Surgical specimens were subjected to dehydration in alcohol, immersion in xylene, and embedding in paraffin. For the immunohistochemical reaction, the sections were deparaffinized with xylene and rehydrated with ethanol solutions. Antigen recovery was performed in sodium citrate pH 6.0. After blocking endogenous peroxidase (in peroxide buffer) and possible nonspecific binding sites of the primary antibody (1% bovine serum albumin), tissue sections were incubated overnight with primary polyclonal anti- Twist1 antibody (H-81; Santa Cruz Biotechnology, Santa Cruz, CA), 1/50 dilution and, after washing, incubated with the second antibody (Kit Super ABC, EASYPath). The third incubation was performed with the avidin-biotin-peroxidase complex (Kit Super ABC, EASYPath), followed by development with diaminobenzidine. The sections were counterstained with Mayer’s hematoxylin for 30 seconds, washed with running water, dehydrated, and finished with Entellan®.
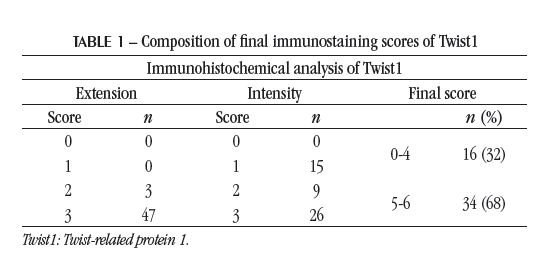
In the immunostaining analysis, each sample was evaluated by a pathologist blinded as to the extension (area) and intensity, as previously described(12). In the extension analysis, the total area of the slide was observed, which presented positive immunostaining, classified as 0 (0%), 1 (1%-30%), 2 (31%-60%), and 3 (61%-100%). Regarding the intensity, it was classified as 0 (negative), 1 (weak), 2 (medium), and 3 (strong). For the final result, a score from 0 to 6 was calculated, obtained by the sum of the extension and intensity scores observed in each sample. Samples with a final score 0 to 4 were classified as normal expression, and scores 5 and 6 as Twist1 overexpression.
Statistical analysis
The Twist1expression profile was described through percentage and absolute frequency. Comparison of Twist1 expression and clinicopathological parameters was investigated by chi-square test or Fisher’s exact test, when appropriate. A p-value lower than 0.05 was considered statistically significant.
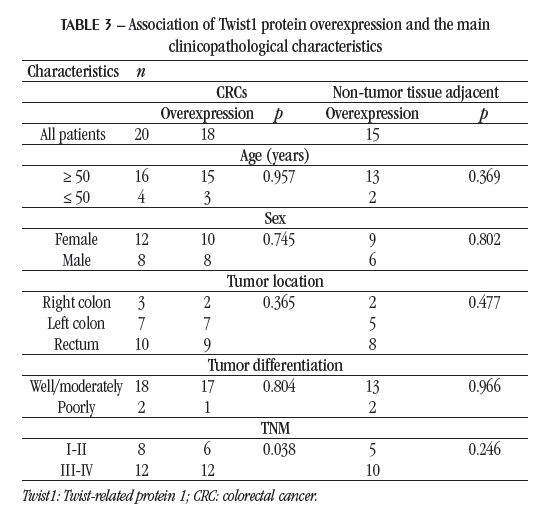
RESULTS The positive immunostaining of Twist, predominantly cytoplasmic, is evidenced by the appearance of brownishbrown granules present among the nuclei counterstained with hematoxylin (Figure). In the global analysis of the set of 50 samples included in the study, there were differences in immunostaining regarding the extension and intensity, as shown in Table 1. Twist1 overexpression was evidenced in 34 (68%) of the 50 samples.
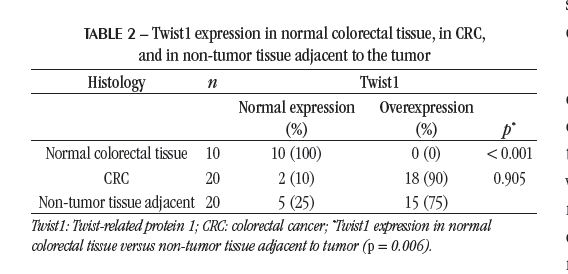
In the cancer patients group, paired samples of tumor and non-tumor tissue from an area located approximately 10 cm from the tumor were analyzed. Twist1 overexpression was observed in 18 of 20 tumor samples and 15 of 20 non-tumor tissue adjacent to the tumor samples. Although they did not differ from each other, both results were statistically significant when compared with non-malignant colorectal tissue samples from control group patients (p < 0.001), in which Twist1 overexpression was not detected (Table 2). In 14 cancer patients, Twist1 overexpression was detected in both tumor tissue and adjacent non-tumor tissue; in four, overexpression was detected only in the tumor; in one, only in adjacent non-tumor tissue; and in one, there was no overexpression in the tumor or the adjacent non-tumor tissue.
The clinical and pathological characteristics of CRC cases were analyzed for their association with Twist1 overexpression (Table 3). Twist1 overexpression is less frequently detected in stage I and II tumors when compared to more advanced stage tumors (p < 0.05). No significant differences were observed regarding the age and gender of the patients, as well as the location and degree of tumors histological differentiation.
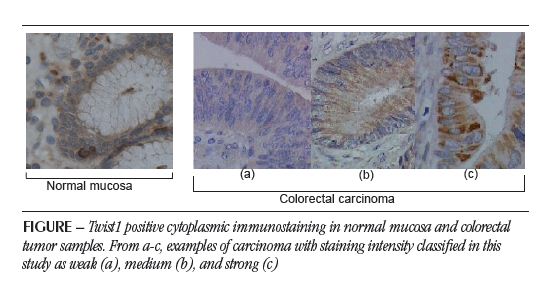
In this study, we analyzed the expression of Twist1 in tumors and adjacent normal tissues, as well as the relationship between overexpression of the Twist1 protein and clinicopathological parameters of patients with CRC. We observed that the Twist1 occurrence is cytoplasmic, with or without nuclear expression, and the overexpression of this protein is exclusive to tumor tissues, not occurring in the normal colon mucosa of patients without CRC, indicating it as a potential tumor marker.
New reports aim to contribute to the definition of the biological role of Twist1 in CRC. Previous studies have evidenced the contribution of this protein in tumor development and metastasis, as well as its influence on the prognosis of carrier patients(10, 13, 14, 18). It is known that Twist1 plays a central role in EMT, a process that leads to changes in polarity, intercellular adhesion molecules, and cell mobility. EMT is initiated when proteins such as N-cadherin, Twist1, and fibronectin are overexpressed, while EMT inhibitors such as E-cadherin and cytokeratins have their expression decreased(3).
The present study is the first to investigate the Twist1 overexpression in patients with CRC, comparing them to the control group without cancer. Our data demonstrate that the Twist1 expression was elevated in 18 samples from patients with a confirmed diagnosis of CRC but in none of the tissues in the non-malignant control group (p < 0.001). The absence of Twist overexpression in the control group suggests that this alteration may represent an essential cofactor in the development of CRC and not a merely incidental finding.
We also observed that in patients with CRC, Twist1 overexpression occurs both in tumor cells and in the mucosa that still does not show visible macro- or microscopic changes. This finding had already been described in other neoplasms(7, 8, 11, 12) and other studies with CRC(14, 15, 18, 19). The detection of overexpression in the normal mucosa of patients with CRC suggests that this may be an early event in the process of colorectal carcinogenesis. Kyo et al. (2006)(12), when analyzing Twist1 in endometrial tumors, found expression in the margins of tumor foci and, in some cases, in the strongly stained histologically normal stroma. One explanation for these findings is that such cells, despite being morphologically similar to the normal stroma, could already present genetic or epigenetic changes, or be derived from cancer cells. Furthermore, the Twist1 overexpression, in the margins of tumoral foci and tissues adjacent to the tumors, may be a consequence of intratumoral hypoxia(6).
However, there is stillcontroversy in the literature over howthisexpression is changed as the diseaseprogresses. A studysimilartooursconductedby Gomez et al. (2011)(14) revealedthat the Twist1 overexpression was restrictedto tumor tissues (86.1%), andnormalmucosa was not found in patientswithCRC. These data togetherindicatethat, although Twist1 overexpression is a more frequent eventconcerningdiseaseprogression (normalmucosa > normaladjacentmucosa > tumor), the exact knowledgeabout the meaning of thisbiologicalfindingremainsunclear.
In this study, the positive reaction and interaction between Twist1 and the anti-Twist1 antibody with the second antibody were predominantly observed in the cytoplasm of epithelial cells. This finding is in line with that observed in the previous study(16), which also verified the cytoplasmic location of Twist1 in CRC. Rauof et al. (2021)(18) reported the cytoplasmic and nuclear localization of Twist1 in CRC, despite noting that this nuclear expression is confined to higher TNM stages tumors, as a late alteration in CRC carcinogenesis. This difference could be explained by our small sample size, deserving further confirmation.
This work demonstrated that Twist1 overexpression in CRC is significantly related to higher TNM stages, as evidenced in previous studies(13, 15, 16, 20). Regarding Twist1 in CRC, previous studies have demonstrated its role in carcinogenesis, such as the induction of EMT in cells in vitro(16). Consistent with these results, we demonstrate here that the relationship between Twist1 overexpression and high TNM stages suggests the involvement of Twist1 in the processes of invasion and metastasis in CRC. However, we did not find a clear correlation between overexpression and other relevant clinicopathological parameters in CRC. Our data do not confirm the finding that Twist1 overexpression is related to low tumor differentiation, as observed by some authors(18, 21). In addition, we also did not observe a relationship between Twist1 overexpression and gender and age, which corroborates the results of previous studies(16, 20), but this is in disagreement with the study by Valdez-Mora et al. (2009)(15), who found significantly higher Twist1 expression in men. These differences can be attributed to the determination of the Twist1 expression level [messenger ribonucleic acid (mRNA)] by polymerase chain reaction (PCR) in real-time or to different cut-offs of the analyzed variables.
There seems to be evidence of the relationship between Twist1 overexpression and reduced survival rate(13, 14, 22, 23) and the acquired resistance to chemotherapy(18), which interfere in the prognosis of patients with CRC. However, the absence of follow-up data in our study did not allow us to analyze the influence of Twist1 overexpression on the clinical course of patients and how it affects the overall survival and disease-free survival of patients with CRC. Additional studies that assess the influence of Twist1 overexpression on prognosis are required.
CONCLUSION
Twist1 is frequently overexpressed in tumor tissue and the normal mucosa adjacent to the tumor of patients with CRC, but it is not found in patients without the neoplasm. This finding may indicate the early involvement of Twist1 in CRC, carcinogenesis, suggesting its possible application as a tumor marker. Twist1 is also associated with the clinical course of CRC, as it is significantly more expressed in tumors at higher TNM stages. However, it should be noted that these observations deserve to be confirmed in studies that analyze a larger number of individuals and clarify the molecular pathways responsible for the alterations observed here, aiming at the application of Twist1 as a diagnostic or prognostic biomarker in CRC.
ACKNOWLEDGEMENT
The authors are grateful to Rosalva Meurer for her technical support.
REFERENCES
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019; 144(8): 1941-53.
- Brasil. Ministério da Saúde. Instituto Nacional do Câncer (Inca). Câncer de intestino [Internet]. Inca [cited July 27, 2020]. Available at: https://www. inca.gov.br/sites/ufu.sti.inca.local/files//media/document//estimativa-2020-incidencia-de-cancer-no-brasil.pdf.
- Zhu QQ, Ma C, Wang Q, Song Y, Lv T. The role of TWIST1 in epithelial-mesenchymal transition and cancers. Tumor Biology. 2016; 37(1): 185-97.
- Yang J, Mani SA, Donaher JL, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004; 117(7): 927-39.
- Yang J, Mani SA, Weinberg RA. Exploring a new twist on tumor metastasis. Cancer Res. 2006; 66(9): 4549-52.
- Yang MH, Wu MZ, Chiou SH, et al. Direct regulation of TWIST by HIF-1α promotes metastasis. Nat Cell Biol. 2008; 10(3): 295-305.
- Rosivatz E, Becker I, Specht K, et al. Differential expression of the epithelial-mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol. 2002; 161(5): 1881-91.
- Yusufu A, Shayimu P, Tuerdi R, Fang C, Wang F, Wang H. TFF3 and TFF1 expression levels are elevated in colorectal cancer and promote the malignant behavior of colon cancer by activating the EMT process. Int J Oncol. 2019; 55(4): 789-804.
- Gort EH, Suijkerbuijk KP, Roothaan SM, et al. Methylation of the TWIST1 promoter, TWIST1 mRNA levels, and immunohistochemical expression of TWIST1 in breast cancer. Cancer Epidemiol Biomarkers Prev. 2008; 17(12): 3325-30.
- Okada T, Suehiro Y, Ueno K, et al. TWIST1 hypermethylation is observed frequently in colorectal tumors and its overexpression is associated with unfavorable outcomes in patients with colorectal cancer. Genes Chromosomes Cancer. 2010; 49(5): 452-62.
- Kwok WK, Ling MT, Lee TW, et al. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer Res. 2005; 65(12): 5153-62.
- Kyo S, Sakaguchi J, Ohno S, et al. High Twist expression is involved in infiltrative endometrial cancer and affects patient survival. Hum Pathol. 2006; 37(4): 431-8.
- Yusup A, Huji B, Fang C, et al. Expression of trefoil factors and TWIST1 in colorectal cancer and their correlation with metastatic potential and prognosis. World J Gastroenterol. 2017; 23(1): 110-20.
- Gomez I, Peña C, Herrera M, et al. TWIST1 is expressed in colorectal carcinomas and predicts patient survival. PLoS One. 2011; 6(3): e18023-e18023.
- Valdés-Mora F, Gómez del Pulgar T, Bandrés E, et al. TWIST1 overexpression is associated with nodal invasion and male sex in primary colorectal cancer. Ann Surg Oncol. 2009;
- (1): 78-87. 16. Zhu DJ, Chen XW, Zhang WJ, et al. Twist1 is a potential prognostic marker for colorectal cancer and associated with chemoresistance. Am J Cancer Res. 2015; 5(6): 2000-11.
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th edition. France: Springer; 2010.
- Raouf SM, Ibrahim TR, Abdelaziz LA, Farid MI. Prognostic value of TWIST1 and EZH2 expression in colon cancer. J Gastrointest Cancer. 2021; 52(1): 90-8.
- Celesti G, Di Caro G, Bianchi P, et al. Presence of Twist1-positive neoplastic cells in the stroma of chromosome-unstable colorectal tumors. Gastroenterology. 2013; 145(3): 647-57.e15.2.
- Kim YH, Kim G, Kwon C, Kim JW, Park PW, Hahm KB. TWIST1 and SNAI1 as markers of poor prognosis in human colorectal cancer are associated with the expression of ALDH1 and TGF-β1. Oncol Rep. 2014; 31(3): 1380-8.
- Hom R, Lim S. Overexpression of Twist1 in colorectal adenocarcinoma. Basic Appl Pathol. 2009; 2(1): 15-20.
- Song LB, Liao WT, Mai HQ, et al. The clinical significance of twist expression in nasopharyngeal carcinoma. Cancer Lett. 2006; 242(2): 258-65.
- Zhu B, Wang Y, Wang X, et al. Evaluation of the correlation of MACC1, CD44, Twist1, and KiSS-1 in the metastasis and prognosis for colon carcinoma. Diagn Pathol. 2018; 13(1): 45.