Effectiveness of novel biomarkers of acute kidney injury in critically ill patients:
a systematic review
Akeme Laissa N. Coutinho1; Djalma G. Xavier Filho1; Marcel Christian M. Tenório2; Matheus R. Lopes1; Adirlene P. O. Tenório1,2
1Universidade Federal do Vale do São Francisco (UNIVASF), Paulo Afonso, Bahia, Brazil.
2Clínica de Doenças Renais do Vale do São Francisco (CLIRENAL), Paulo Afonso, Bahia, Brazil.
J Bras Patol Med Lab. 2021; 57: 1-10.
DOI: 10.5935/1676-2444.20210059
Correspondence Author
Matheus Rodrigues Lopes
ORCID 0000-0002-3719-4131
e-mail: matheuslopesbio@gmail.com
First Submission on 02/09/21
Last Submission on 02/11/21
Accepted for publication on 08/14/21
Published on 12/20/21
ABSTRACT
Introduction: Acute kidney injury (AKI) is defined as an abrupt decrease in the glomerular filtration rate in a short period of days or hours. Due to the limitations of current renal markers, several studies have sought to determine new markers for the early detection of AKI. Objective: To evaluate the effectiveness of novel biomarkers in diagnosing AKI in critically ill patients. Methods: Systematic literature review carried out using the MEDLINE databases via PubMed and Scielo. Observational original articles of the last five years that presented the most current and available evidence on novel biomarkers of AKI, were included. The search for references was limited to studies published in Portuguese and English. The keywords used were acute kidney injury, biomarkers, and intensive care units and their respective translations into Portuguese. Results: 155 articles were identified, of which 19 were included in this review. The neutrophil gelatinase-associated lipocalin (NGAL) showed superiority in relation to serum creatinine in AKI due to its rapid elevation regarding the injury. The serum and urine fractions of kidney injury molecule-1 (KIM-1) have a good predictive value for AKI, but they are not useful for estimating the severity of the injury. The product between tissue inhibitor of metalloproteinases 2 (TIMP-2) and insulin-like growth factor-binding protein 7 (IGFBP7) proved to be superior to other biomarkers in the AKI diagnosis. Cystatin C is less influenced by individual parameters compared to serum creatinine. Discussion: Several studies prove the effectiveness of the main markers in the early diagnosis of AKI and in predicting the outcome of the disease in critically ill patients. The combination of markers can increase the predictive value for the AKI diagnosis.
Key words: acute kidney injury; biomarkers; intensive care units; critical care; nephrology.
RESUMO
Introdução: A injúria renal aguda (IRA) é definida como redução abrupta da taxa de filtração glomerular em um curto período de dias ou horas. Em razão das limitações dos marcadores renais atuais, diversos estudos buscam determinar novos marcadores para a detecção precoce da IRA. Objetivo: Avaliar a eficácia dos novos biomarcadores no diagnóstico de IRA em pacientes críticos. Métodos: Revisão sistemática da literatura realizada por meio das bases de dados MEDLINE via PubMed e Scielo. Foram incluídos artigos originais observacionais dos últimos cinco anos que apresentassem as evidências disponíveis e mais atuais sobre novos biomarcadores da injúria renal. A busca de referências se limitou a estudos publicados em língua portuguesa e língua inglesa. Os descritores utilizados foram injúria renal aguda, biomarcadores e unidades de terapia intensiva e suas respectivas traduções em língua inglesa. Resultados: Foram identificados 155 artigos, sendo 19 incluídos nesta revisão. A neutrophil gelatinase associated lipocalin (NGAL) demonstrou superioridade em relação à creatinina sérica na IRA por sua rápida elevação frente ao insulto. As frações sérica e urinária de kidney injury molecule-1 (KIM-1) possuem bom valor preditivo para IRA, mas não são úteis para estimar a gravidade da injúria. O produto entre tissue inhibitor of metalloproteinases 2 (TIMP-2) e insulin-like growth factor-binding protein 7 (IGFBP7) demonstrou ser superior a outros biomarcadores no diagnóstico da IRA. A cistatina C é menos influenciada por parâmetros individuais se comparada com a creatinina sérica. Discussão: Diversos estudos comprovam a eficácia dos principais marcadores no diagnóstico precoce da IRA e na predição do desfecho da doença em pacientes críticos. A combinação entre marcadores pode ampliar o valor preditivo para o diagnóstico da IRA.
Unitermos: lesão renal aguda; biomarcadores; unidades de terapia intensiva; cuidados críticos; nefrologia.
RESUMEN
Introducción: La insuficiencia renal aguda (IRA) se define como una reducción abrupta de la tasa de filtración glomerular en un período corto de días u horas. Debido a las limitaciones de los marcadores renales actuales, varios estudios han buscado determinar nuevos marcadores para la detección precoz de la IRA. Objetivo: Evaluar la efectividad de nuevos biomarcadores en el diagnóstico de IRA en pacientes críticos. Métodos: Revisión sistemática de la literatura utilizando las bases de datos MEDLINE a través de PubMed y SciELO. Se incluyeron artículos observacionales originales de los últimos cinco años que presentaban la evidencia más actual y disponible sobre nuevos biomarcadores de IRA. La búsqueda de referencias se limitó a estudios publicados en portugués e inglés. Los descriptores utilizados fueron: acute kidney injury, biomarkers y intensive care units y sus respectivas traducciones al portugués. Resultados: Se identificaron 155 artículos, de los cuales 19 fueron incluidos en esta revisión. La lipocalina asociada a la gelatinasa de neutrófilos (NGAL) mostró superioridad en relación con la creatinina sérica en la IRA debido a su rápido aumento con respecto a la lesión. Las fracciones de suero y orina de la molécula-1 de lesión renal (KIM-1) tienen un buen valor predictivo de IRA, pero no son útiles para estimar la gravedad de la lesión. El producto entre el inhibidor tisular de la metaloproteinasa-2 (TIMP-2) y la proteína-7 de unión al factor de crecimiento similar a la insulina (IGFBP7) demostró ser superior a otros biomarcadores en el diagnóstico de IRA. La cistatina C está menos influenciada por los parámetros individuales en comparación con la creatinina en suero. Discusión: Diversos estudios demuestran la efectividad de los principales marcadores en el diagnóstico precoz de la IRA y en la predicción de la evolución de la enfermedad en pacientes críticos. La combinación de marcadores puede aumentar el valor predictivo para el diagnóstico de IRA.
Palabras clave: insuficiencia renal aguda; biomarcadores; unidades de cuidados intensivos; cuidado crítico; nefrología.
INTRODUCTION
Acute kidney injury (AKI) is defined as an abrupt decrease in the glomerular filtration rate (GFR) in a short period of days or hours. It represents a decrease in the renal excretion function and, consequently, the retention of nitrogenous waste and other products that, under normal conditions, would be physiologically eliminated by the kidneys(1). AKI is known for its multifactorial etiology and among its main causes are sepsis, major surgery, cardiogenic shock, hypovolemia, and drug-induced nephrotoxic insults(2).
The AKI incidence represents approximately ⅔ of all patients admitted to intensive care units (ICU) and about 10% of hospital admissions(3). Therefore, AKI is presented as a relevant public health problem worldwide, associated not only with an increase in morbidity and mortality but with an increase in the time and costs of hospitalization(4).
The most commonly used AKI classification is currently defined by the Kidney Disease: Improving Global Outcomes (KDIGO) group, which is based on serum creatinine (sCr) levels and urine output. This criterion defines the AKI as an increase in creatinine value by ≥ 0.3 mg/dl in 48 hours, a 50% increase in sCr compared to baseline in seven days, or a reduction in urine output to < 0.5 ml/kg/h for a period longer than six hours(5).
Despite its low cost and easy laboratory application, creatinine measurement has limitations that prevent it from being a good marker for the early identification of AKI, mainly due to the fact that the changes in its levels occur later in relation to renal injury(6). The time between kidney damage and creatinine elevation can vary between two days and one week. Furthermore, for minimal sCr increase, it may be necessary to reduce GFR by 50% of normal renal function(7). Moreover, some factors can interfere with creatinine levels without causing kidney damage, such as ethnicity, meat consumption, medication use, sepsis, and liver diseases(8).
In hospitalized patients, oliguria presents with relative sensitivity and low specificity for AKI. Clinical conditions, such as transient hemodynamic instability, can lead to decreased diuresis without necessarily denoting acute kidney injury. Besides, the kidneys may be in a harmful process with not necessarily presenting oliguria(9). Urine output can also be interfered with by the use of diuretics, medications commonly used in critically ill patients(10).
Due to these limitations, several studies have sought to determine new markers for the early detection of AKI. Among the main biomarkers studied are: neutrophil gelatinase associated lipocalin (NGAL), kidney injury molecule-1
(KIM-1), tissue inhibitor of metalloproteinases 2 (TIMP-2), insulin-like growth factor-binding protein 7 (IGFBP7), and cystatin C. The assessment of the general ability of one of these biomarkers to predict AKI is usually performed using the following tools: receiver operating characteristic (ROC curve) and area under the curve (AUC). It is understood that markers with higher AUC values also have greater predictive value for AKI(11).
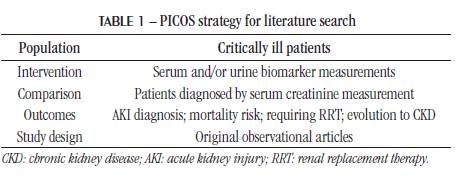
This study aimed to evaluate the effectiveness of novel biomarkers in the diagnosis of AKI in critically ill patients. Despite advances in proving the effectiveness of several biomarkers, there are still characteristics that need to be defined for their inclusion in clinical practice, such as the optimum time to measure them, as well as their efficiency in different populations. Furthermore, to date, no other systematic reviews addressing the main markers of AKI in critically ill patients have been found. METHODS This systematic literature review aimed to answer the following question: are new biomarkers efficient in diagnosing AKI earlier than serum creatinine? The research question was developed using the PICOS strategy (Table 1). Eligibility criteria Only original observational articles were included, from 2015 to 2020, which brought together the available and most current evidence on the contribution of new biomarkers for AKI diagnosis in critically ill patients. The search for references was limited to studies published in Portuguese and English. To be included, the studies must be on AKI diagnosis through serum creatinine measurement. The new biomarkers could be measured in serum and/or urine samples. Those in which the outcomes analyzed by the researchers were related to the presence, severity, and/or progression of the disease were selected.
Exclusion criteria
Studies focusing exclusively on chemical aspects of biomarkers and not involving human beings, those in which the patients involved were not in a hospital environment or intensive care units, and articles not available in the full version, were excluded. Studies that did not analyze the outcomes of interest in this study, studies of low methodological quality, unpublished manuscripts, and conference abstracts were considered ineligible.
Study search and selection strategy
A bibliographic survey was carried out using the MEDLINE databases via PubMed and SciELO until September 31, 2020. The search strategy included the descriptors and terms translated into The search strategy included the descriptors and terms translated into Portuguese in acute kidney injury, biomarkers, and intensive care units. The descriptors used in the English language appear in the Medical Subject Headings Terms (MeSH) of the US National Library of Medicine (NLM). These descriptors were used individually or together through Boolean operators (and; or).
The example of the search strategy used for PubMed are shown in Table 2.
The search was initially carried out using descriptors and their respective translations, directly in the referred databases. In the second stage of selection, the date restriction was carried out for articles published from the year 2015 onwards, to identify the most recent literature on this topic.

Quality assessment of studies
The assessment of methodological quality was performed descriptively and independently by two reviewers. The titles and abstracts identified in the search strategy were analyzed, according to the inclusion and exclusion criteria, by both reviewers independently.
In the subsequent step, the complete reading of the selected articles and the application of QUADAS-2 were carried out. This tool consists of the analysis of four domains: patient selection, index test, reference and flow/synchrony test. For each domain, questions are provided and can be answered with yes, no, or unclear. In the end, the risk of bias and applicability are judged based on the set of information obtained from the data diagram(12).
In cases of disagreement or the presence of papers that had unclear information in at least one questioning of the QUADAS-2, a consensus was sought between the two reviewers. Articles that did not present the information required by the four QUADAS-2 assessment domains and/or considered of low methodological quality by both reviewers were excluded from the analysis.
Data extraction and analysis
Data were independently extracted by the reviewers, using standardized key questions that listed: the main biomarkers for AKI, the study description, the characterization of the patients involved, and the main results and conclusions verified. Data analysis was performed descriptively.
RESULTS
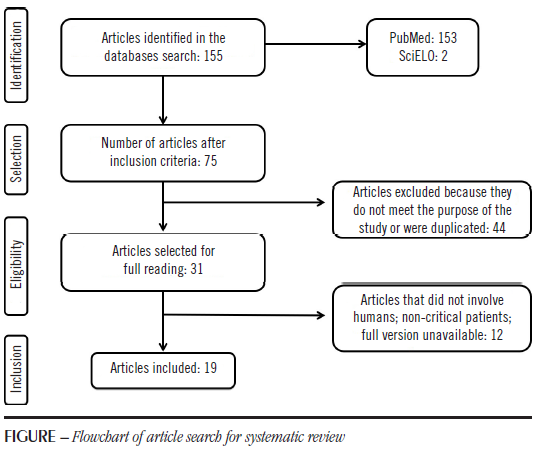
The initial search, based on the descriptors in English, resulted in 155 articles, 153 of which were found in PubMed, and only two were provided by SciELO. When using the descriptors in Portuguese, no additional studies were found, which indicates a lack of national research on the subject and a need for scientific studies on the use of new biomarkers in the country. Then, the period for articles published was restricted for the last five years, which resulted in 75 studies. After reading the abstracts, 44 articles were excluded because they were not suitable to this study objective, or were duplicated. From the remaining 31, 12 were discarded after reading the articles in full and applying the exclusion criteria. Thus, the final sample of 19 articles was reached. The flowchart describing the search for articles is presented in the Figure.

From the 19 articles selected, 18 were observational studies and one was a secondary analysis of an observational study. Most studies were carried out in the Asian continent (43.37%), followed by the European continent (36.84%), Oceania (5.26%), Africa (5.26%), and America (5, 26%). The biomarkers that concentrated the greatest number of studies were TIMP-2 and IGFBP7 (36.84%), followed by NGAL and cystatin C with the same number of studies (26.32%) and, finally, KIM-1 (10.52%). The main advantages and disadvantages of these biomarkers are shown in Table 3.
Among the final sample, most studies were carried out in individuals older than 18 years old (84.21%), while the remaining presented children and/or neonates as the target population

(15.79%). The causes of hospitalization varied between clinical and surgical contexts, with emphasis on patients with sepsis and individuals undergoing cardiac surgery.
The KDIGO diagnostic criterion was used alone by 85.7% of the studies, the other studies (14.3%) also adopted other classifications for AKI, such as: acute kidney injury network (AKIN), and risk, injury, failure, Loss of kidney function, Endstage kidney disease (RIFLE). Regarding the performance of the investigated markers, the studies used the parameters of sensitivity and specificity, as well as the precision technique of the AUC-ROC test. The main characteristics of the included studies are shown in Table 4.
NGAL
In a cross-sectional observational study, Shoaib et al. (2019)(28) found that the accuracy of urine NGAL in the diagnosis of AKI was 90.7% in adult patients admitted to ICU. They also observed that the increase in NGAL levels can precede changes in serum creatinine by up to two days in cases of kidney injury.
Pejović et al. (2015)(27) aimed to prove the usefulness of NGAL in the early diagnosis of AKI in a different age group by conducting a study with premature neonates diagnosed with perinatal asphyxia. In this case, it was observed that the measurement of serum NGAL concentration in the first four hours of the first day of life is predictive of the onset and severity of AKI in these patients.
Fanning et al. (2016)(17) performed serial measurements of urine and serum NGAL on 18 occasions over the first 48 hours after cardiac surgery in patients at high risk for kidney damage. It was found that urine collections performed at two times, four and 24 hours after the installation of cardiopulmonary bypass (CPB), showed a greater predictive value of NGAL in identifying AKI compared to other periods. When comparing the performance of the two NGAL fractions, it was identified that the urine measurement of this marker performed better than the serum fraction.
Introcaso et al. (2018)(21), when analyzing the use of NGAL as a marker for kidney injury in patients undergoing cardiac surgery, they found that the consecutive measurement of plasma NGAL before and after the surgical procedure can predict AKI in these patients. Post-surgical NGAL values had an area under the ROC curve of 0.71; 95% CI (0.60-0.82); and 76% sensitivity and 59% specificity for 154 ng/ml cutoff. When using two consecutive measurements of NGAL, the sensitivity increased to 86%. Furthermore, it was found that the combination of the measurement of this biomarker with the measurement of serum creatinine levels reinforces the diagnosis of kidney injury, which establishes an increase in both sensitivity and specificity.
Aiming to evaluate the use of novel AKI biomarkers, considering the extra-renal conditions and the severity of the systemic involvement of patients, Asada et al. (2016)(13) found that urine NGAL has a linear correlation with two important inflammatory markers: C-reactive protein and white blood cell count. Moreover, combining the NGAL with the Acute Physiology and Chronic Health disease Classification System II (APACHE II) score and the patient’s clinical condition has been found to improve its predictive value for AKI. This combination demonstrated a better area under the ROC curve (AUC-ROC 0.940) than the use of NGAL alone (AUC-ROC 0.858).
KIM-1
By following patients diagnosed with sepsis, Zhang et al. (2020)(31) identified that urine KIM-1 levels were higher in septic patients who developed AKI than in those who did not progress to the disease. When measuring serum KIM-1 levels, the group of patients with sepsis and AKI showed higher levels of this marker compared to septic patients without AKI. Thus, both fractions of this marker showed good predictive value for AKI. However, they did not prove to be useful for estimating disease severity.
Khreba et al. (2019)(22) developed a prospective cohort study with patients undergoing cardiac surgery with extracorporeal circulation (ECC). It was found that the urine measurement of this biomarker, three hours after the surgery, showed significantly higher levels in patients who developed AKI compared to the group who did not develop the disease. The measurement of urine KIM-1 within three hours after the procedure showed a sensitivity of 48% and a specificity of 94%.
TIMP-2 and IGFBP7
Through the analysis of the urine fractions of TIMP-2 and IGFBP7 from patients with septic shock, Maizel et al. (2019)(26) found that the result of the product between the concentrations of these biomarkers has shown significant predictive value for the onset of moderate or severe AKI (stages two and three of KDIGO, respectively) within 24 hours. Furthermore, it was identified that a score > 2 (ng/ml)²/1,000 as a result of the equation [TIMP-2]•[IGFBP7] reflects a four times higher risk of AKI stage three of KDIGO, within 24 hours.
Other observational studies, such as those by Di Leo et al. (2018)(25) and Ferrari et al. (2019)(18), used the cutoff of 0.3 (ng/ml)2/1,000 and found that values above this point represent a seven times greater risk for the onset of AKI compared to lower scores. Maizel et al. (2019)(26) recognized that, depending on the cutoff point used, the sensitivity and specificity parameters for these biomarkers can be altered: 92% sensitivity and 46% specificity for the 0.3 cutoff, and 37% of sensitivity and 95% specificity for the 2 cutoff.
Wang et al. (2017)(29) investigated the use of [TIMP-2]•[IGFBP7] for early identification of acute injury in critically ill patients undergoing cardiac surgery. This allowed us to identify that [TIMP-2]•[IGFBP7] results with urine samples collected four hours after admission to the ICU were significantly higher in patients who developed AKI than in those who did not have the disease (p < 0.001).
Koyner et al. (2015)(23) demonstrated the usefulness of these two markers for the prognosis of other relevant disease outcomes. The use of only one measure of [TIMP-2]•[IGFBP7] has shown considerable value in identifying increased risk of requiring renal replacement therapy (RRT) and mortality within up to nine months in a critically ill adult population. In a prospective cohort study in the same year, Westhoff et al. (2015)(30) found a good prognostic value of markers for the outcome of death and moderate performance for the RRT initiation in a different age group, when studying neonates and children diagnosed with AKI. The study by Cho et al. (2020)(14) strengthens these findings and points out the importance of [TIMP-2]•[IGFBP7] to predict an individual’s renal recovery, as well as their risk of progressing to CKD (chronic kidney disease).
Cystatin C
Leem et al. (2017)(24) showed that septic patients who developed AKI had significantly higher serum cystatin C values compared to those who did not have the disease. The measurement of this biomarker at admission to the ICU correlated well with the onset of AKI and was associated with later recovery from renal involvement.
Hošková et al. (2016)(19), in a prospective study with patients undergoing heart transplantation, they found the usefulness of cystatin C as an early marker of AKI up to three hours after surgery (levels > 1.6 mg/l showed a predictive value for AKI). Furthermore, it was identified that persistently elevated cystatin C levels (> 2.54 mg/l on the seventh day after transplantation) can predict mortality within one year.
Hu et al. (2017)(20) also recognized the prognostic ability of cystatin C for patients admitted to a coronary care unit. This marker was able to predict the risk of mortality in up to two years, as well as the possibility of patient readmission and failure of renal function recovery for those with serum Cys C values ≥ 1.255 mg/l.
Deng et al. (2017)(15) demonstrated that serum Cys C had a considerably higher area under the ROC curve values when compared with another renal biomarker [N-acetyl-β-Dglucosaminidase (NAG)] and with the albumin-creatinine ratio. This study showed that the combination of serum cystatin C and urine NAG has greater potential for the early diagnosis of AKI than the measurement of these markers alone.
In a study with newborns and children in critical conditions, Fang et al. (2018)(16) recognized the superiority of cystatin C over sCr by showing that the urine fraction of Cys C increases from one to two days before the creatinine changes in the AKI. Furthermore, in agreement with previous studies, it was observed that urine C cystatin is a good marker for predicting mortality in these age groups. It was found that for every 1% increase in urine cystatin values, the probability of death outcome increases by 26%. When applying the cutoff point of 1.260 ng/mg, urine Cys C presented 79.2% sensitivity and 72.3% specificity.
DISCUSSION
Numerous studies aim to the development of novel biomarkers for the AKI detection in early stages. The main current markers are related to the pathophysiology of the disease, which ranges from situations of stress and/or renal injury to elements associated with checkpoints in the cell cycle and inflammatory pathways(32). It is believed that the new markers can identify kidney insults in preclinical and sub-clinical phases, which can allow the establishment of early treatment for the individual and the prevention of further kidney damage(33).
NGAL is a 25 kDa glycoprotein of the lipocalin family, initially identified in neutrophils, but it can also be found in renal tubule cells(34). Unlike serum creatinine, which is used as an indicator of renal function, changes in serum and urine NGAL levels suggest direct kidney damage, since this protein is released during the chronic or acute development of tubular injury. As observed in this study, another factor that suggests the advantage of NGAL over sCr in AKI is its rapid increase in view of injury(27).
Although NGAL proves to be a good biomarker for AKI in different age groups, the ideal time to perform the measurements is still not a consensus. Based on the research described in this study, it is inferred that the measurement of serum and urine NGAL fractions in the first four hours after the onset of the lesion is able to identify changes suggestive of AKI development(17, 21).
Certain clinical contexts influence the predictive power of NGAL, especially in patient with a systemic inflammatory condition. It is understood that the combination of NGAL with other renal markers and the clinical status significantly increases its predictive value for AKI, which reinforces the importance of recognizing the patient’s extrarenal conditions(31).
The marker with the fewest described studies was KIM-1, a transmembrane protein that can be found in epithelial cells of the proximal renal tubule(35). It is known that, during ischemic and/or nephrotoxic processes, the extracellular segment of KIM-1 is released into the renal tubules, increasing its urine levels(36). This glycoprotein has a relevant value as an early biomarker for acute kidney injury, since it is generally not expressed in patients with normal renal function(37).
Despite its efficiency in predicting AKI, KIM-1 did not prove to be useful to estimate the severity of the injury. The likely explanation lies in the fact that this glycoprotein is involved not only in the course of ischemia but also during the repair process of renal tubule cells. Therefore, when identifying high levels of KIM- 1, it is not possible to determine whether it is due to damage or regenerative processes, which is a barrier that prevents to define the severity of the disease(22, 36).
The markers that concentrated the greatest number of studies were TIMP-2 and IGFBP7, two proteins secreted by renal tubule cells as a result of of various cellular insults (ischemia, nephrotoxins, and septic and inflammatory conditions). Both are involved in the arrest of the cell cycle in the G1 phase after the onset of cell damage(2). Due to the favorable results presented by several studies, the use of these markers for patients admitted to ICU was approved by the Food and Drug Administration (FDA), in 2014(38).
Recently, the product between TIMP-2 and IGFBP7 has shown superiority as compared to other renal biomarkers in the diagnosis of acute injury(23, 38). Nevertheless, there is no consensus among researchers regarding the ideal cutoff point for predicting AKI. Higher cutoff values are associated with greater specificity and reduced sensitivity(23). Besides their good performance as early markers for AKI, different studies have shown the effectiveness of TIMP-2 and IGFBP7 in predicting disease outcome (risk of death, requiring RRT, and progression to CKD)(14, 23, 30).
Cystatin C, a low-molecular-weight protein, has shown the ability to act as an early marker of GFR reduction with higher quality than serum creatinine. Compared with sCr, cystatin C is less influenced by individual parameters, such as age, gender, and muscle mass(39). During the early phase of AKI, the serum concentration of cystatin C increases more rapidly compared to the increase in creatinine, since the former has a significantly shorter half-life(40). This biomarker also has a prognostic value for AKI and, when used in combination with another marker, significantly increases its predictive value for the onset of the disease(15).
Overall, the analyzed studies demonstrate the good performance of renal biomarkers in the early diagnosis of AKI, as well as in estimating the severity and prognosis of the disease. Among the main disadvantages described in most studies is the lack of consensus on the optimal time to marker measurement and the cutoff point for its analysis.
A relevant limitation of this study was the lack of studies carried out in the national scenario, which prevented the finding and discussion of studies on novel renal biomarkers in the country. Furthermore, the variety between the clinical conditions of the study individuals and the samples obtained are other limitations found.
CONCLUSION
There has been an important recent advance regarding the use of novel renal biomarkers in the AKI scenario. Several studies have proven the effectiveness of the main markers both in the early diagnosis of acute injury and in predicting the outcome of the disease in critically ill patients. It was possible to verify that the combination of different biomarkers proved to be effective to increase the predictive value during the AKI investigation. Furthermore, it is relevant to correlate changes in marker levels with the clinical context presented by the patient.
It is required expanding research in this area, to determine important points for applicability in medical practice, define the optimal time for the biomarkers measurement, and provide standards for their cutoff values.
REFERENCES
1. Forest TW, Colle D. Molécula de injúria renal 1 (KIM-1) no diagnóstico da lesão renal aguda. Rev Cienc Saude. 2019; 31: 74-83. doi: 10.14295/vittalle. v31i1.8372.
2. Nalesso F, Cattarin L, Gobbi L, Fragasso A, Garzotto F, Calò LA. Evaluating Nephrocheck® as a predictive tool for acute kidney injury. Int J Nephrol Renovasc Dis. 2020; 13: 85-96. PubMed PMID: 32425580.
3. Hoste E, Bihorac A, Al-Khafaji A, Ortega LM, Ostermann M, Haase M, et al. Identification and validation of biomarkers of persistent acute kidney injury: the RUBY study. Intensive Care Med. 2020; 46(5): 943-53. PubMed PMID: 32025755.
4. Xie Y, Ankawi G, Yang B, Garzotto F, Passannante A, Breglia A, et al. Tissue inhibitor metalloproteinase-2 (TIMP-2)•IGF-binding protein-7 (IGFBP7) levels are associated with adverse outcomes in patients in the intensive care unit with acute kidney injury. Kidney Int. 2019; 95(6): 1486-93. PubMed PMID: 30982674.
5. Kidney Disease: Improving Global Outcomes Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012; 2 sup: 1-138.
6. Almeida JP, Valente IF, Lordelo MDR. Association between pediatric risk, injury, failure, loss and end stage renal disease score and mortality in a pediatric intensive care unit: a retrospective study. Rev Bras Ter Intensiva. 2018; 30(4): 429-35. PubMed PMID: 30624493.
7. Weiss R, Meersch M, Pavenstädt HJ, Zarbock A. Acute kidney injury: a frequently underestimated problem in perioperative medicine. Dtsch Arztebl Int. 2019; 116(49): 833-42. PubMed PMID: 31888797.
8. Ostermann M, Joannidis M. Acute kidney injury 2016: diagnosis and diagnostic workup. Crit Care. 2016; 20(1): 299. PubMed PMID: 27670788.
9. Lehner GF, Forni LG, Joannidis M. Oliguria and biomarkers of Acute kidney injury: star struck lovers or strangers in the night? Nephron. 2016; 134(3): 183-90. PubMed PMID: 27505252.
10. Gaião SM, Paiva J. Biomarkers of renal recovery after acute kidney injury. Rev Bras Ter Intensiva. 2017; 29(3): 373-81. PubMed PMID: 29044306.
11. Beker BM, Corleto MG, Fieiras C, Musso CG. Novel acute kidney injury biomarkers: their characteristics, utility and concerns. Int Urol Nephrol. 2018; 50(4): 705-13.
12. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine. 2011; 155(8): 529-36. PubMed PMID: 22007046.
13. Asada T, Isshiki R, Hayase N, Sumida M, Inokuchi R, Noiri E, et al. Impact of clinical context on acute kidney injury biomarker performances: differences between neutrophil gelatinase-associated lipocalin and L-type fatty acid-binding protein. Sci Rep. 2016; 6: 33077. PubMed PMID: 27605390.
14. Cho WY, Lim SY, Yang JH, Oh SW, Kim MG, Jo SK. Urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 as biomarkers of patients with established acute kidney injury. Korean J Intern Med. 2020; 35(3): 662-71. PubMed PMID: 31856559.
15. Deng Y, Chi R, Chen S, Ye H, Yuan J, Wang L, et al. Evaluation of clinically available renal biomarkers in critically ill adults: a prospective multicenter observational study. Crit Care. 2017; 21(1): 46. PubMed PMID: 28264714.
16. Fang F, Hu X, Dai X, Wang S, Bai Z, Chen J, et al. Subclinical acute kidney injury is associated with adverse outcomes in critically ill neonates and children. Crit Care. 2018; 22(1): 256. PubMed PMID: 30305134.
17. Fanning N, Galvin S, Parke R, Gilroy J, Bellomo R, McGuinness S. A prospective study of the timing and accuracy of neutrophil gelatinase-associated lipocalin levels in predicting acute kidney injury in high-risk cardiac surgery patients. J Cardiothorac Vasc Anesth. 2016; 30(1): 76-81. PubMed PMID: 26603784.
18. Ferrari F, Romero-González G, Topete LR, Senzolo M, Lorenzin A, Husain-Syed F, et al. Routine adoption of urinary [IGFBP7]∙[TIMP-2] to assess acute kidney injury at any stage 12 hours after intensive care unit admission: a prospective cohort study. Sci Rep. 2019; 9(1): 16484. PubMed PMID: 31712687.
19. Hošková L, Franekova J, Málek I, Kautzner J, Szárszoi O, Jabor A, et al. Comparison of cystatin C and NGAL in early diagnosis of acute kidney injury after heart transplantation. Ann Transplant. 2016; 21: 329-245. PubMed PMID: 27226081.
20. Hu Y, Liu H, Du L, Wan J, Li X. Serum cystatin C predicts AKI and the prognosis of patients in coronary care unit: a prospective, observational study. Kidney Blood Press Res. 2017; 42(6): 961-73. PubMed PMID: 29179178.
21. Introcaso G, Nafi M, Bonomi A, L’Acqua C, Salvi L, Ceriani R, et al. Improvement of neutrophil gelatinase-associated lipocalin sensitivity and specificity by two plasma measurements in predicting acute kidney injury after cardiac surgery. Biochem Med (Zagreb). 2018; 28(3): 030701. PubMed PMID: 30429668.
22. Khreba NA, Abdelsalam M, Wahab AM, Sanad M, Elhelaly R, Adel M, et al. Kidney injury molecule 1 (KIM-1) as an early predictor for acute kidney injury in post-cardiopulmonary bypass (CPB) in open heart surgery patients. Int J Nephrol. 2019; 2019: 6265307. PubMed PMID: 30993020.
23. Koyner JL, Shaw AD, Chawla LS, Hoste EA, Bihorac A, Kashani K, et al. Tissue inhibitor metalloproteinase-2 (TIMP-2)•IGF-binding protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J Am Soc Nephrol. 2015; 26(7): 1747-54. PubMed PMID: 25535301.
24. Leem AY, Park MS, Park BH, Jung WJ, Chung KS, Kim SY, et al. Value of serum cystatin C measurement in the diagnosis of sepsis-induced kidney injury and prediction of renal function recovery. Yonsei Med J. 2017; 58(3): 604-12. PubMed PMID: 28332367.
25. Di Leo L, Nalesso F, Garzotto F, Xie Y, Yang B, Virzì GM, et al. Predicting acute kidney injury in intensive care unit patients: the role of tissue inhibitor of metalloproteinases-2 and insulin-like growth factor-binding protein-7 biomarkers. Blood Purif. 2018; 45(1-3): 270-7. PubMed PMID: 29478052.
26. Maizel J, Daubin D, Vong LV, Titeca-Beauport D, Wetzstein M, Kontar L, et al. Urinary TIMP2 and IGFBP7 identifies high risk patients of short-term progression from mild and moderate to severe acute kidney injury during septic shock: a prospective cohort study. Dis Markers. 2019; 2019: 3471215. PubMed PMID: 31061681.
27. Pejović B, Erić-Marinković J, Pejović M, Kotur-Stevuljević J, Peco-Antić A. Detection of acute kidney injury in premature asphyxiated neonates by serum neutrophil gelatinase-associated lipocalin (sNGAL) – sensitivity and specificity of a potential new biomarker. Biochem Med (Zagreb). 2015; 25(3): 450-9. PubMed PMID: 26525750.
28. Shoaib M, Mahmud SN, Safdar M. Early diagnosis of acute kidney injury by urinary neutrophil gelatinase associated lipocalin in adult critically ill patients. J Ayub Med Coll Abbottabad. 2019; 31(1): 12-5. PubMed PMID: 30868775.
29. Wang Y, Zou Z, Jin J, Teng J, Xu J, Shen B, et al. Urinary TIMP-2 and IGFBP7 for the prediction of acute kidney injury following cardiac surgery. BMC Nephrol. 2017; 18(1): 177. PubMed PMID: 28558754.
30. Westhoff JH, Tönshoff B, Waldherr S, Pöschl J, Teufel U, Westhoff TH, et al. Urinary tissue inhibitor of metalloproteinase-2 (TIMP-2)•insulin-like growth factor-binding protein 7 (IGFBP7) predicts adverse outcome in pediatric acute kidney injury. PLoS One. 2015; 10(11): e0143628. PubMed PMID: 26606754.
31. Zhang CF, Wang HJ, Tong ZH, Zhang C, Wang YS, Yang HQ, et al. The diagnostic and prognostic values of serum and urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin in sepsis induced acute renal injury patients. Eur Rev Med Pharmacol Sci. 2020; 24(10): 5604-17. PubMed PMID: 32495895.
32. Schaub JA, Parikh CR. Biomarkers of acute kidney injury and associations with short-and long-term outcomes. F1000Research. 2016; 5. PubMed PMID: 27239295.
33. Bellomo R, Ronco C, Mehta RL, Asfar P, Boisramé-Helms J, Darmon M, et al. Acute kidney injury in the ICU: from injury to recovery: reports from the 5th Paris International Conference. Ann Intensive Care. 2017; 7(1): 49. PubMed PMID: 28474317.
34. Wu B, Chen J, Yang Y. Biomarkers of acute kidney injury after cardiac surgery: a narrative review. Biomed Res Int. 2019; 2019: 7298635. PubMed PMID: 31346523.
35. Pang HM, Qin XL, Liu TT, Wei WX, Cheng DH, Lu H, et al. Urinary kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin as early biomarkers for predicting vancomycin-associated acute kidney injury: a prospective study. Eur Rev Med Pharmacol Sci. 2017; 21(18): 4203-13. PubMed PMID: 29028077.
36. Song J, Yu J, Prayogo GW, Cao W, Wu Y, Jia Z, et al. Understanding kidney injury molecule 1: a novel immune factor in kidney pathophysiology. Am J Transl Res. 2019; 11(3): 1219-29. PubMed PMID: 30972157.
37. Lei L, Li LP, Zeng Z, Mu JX, Yang X, Zhou C, et al. Value of urinary KIM-1 and NGAL combined with serum cys C for predicting acute kidney injury secondary to decompensated cirrhosis. Sci Rep. 2018; 8(1): 7962. PubMed PMID: 29784944.
38. Fan W, Ankawi G, Zhang J, Digvijay K, Giavarina D, Yin Y, et al. Current understanding and future directions in the application of TIMP-2 and IGFBP7 in AKI clinical practice. Clin Chem Lab Med. 2019; 57(5): 567-76. PubMed PMID: 30179848.
39. Zhang D, Gao L, Ye H, Chi R, Wang L, Hu L, et al. Impact of thyroid function on cystatin C in detecting acute kidney injury: a prospective, observational study. BMC Nephrol. 2019; 20(1): 41. PubMed PMID: 30727972.
40. Che M, Wang X, Xie B, Huang R, Liu S, Yan Y, et al. Use of both serum cystatin C and creatinine as diagnostic criteria for cardiac surgery-associated acute kidney injury and its correlation with long-term major adverse events. Kidney Blood Press Res. 2019; 44(3): 415-25. PubMed PMID: 31189155.




